Patient-derived tumoroids are living 3D models of a patient’s tumor in the lab – tumor twins
These tumor twins retain key characteristics of the original cancer, allowing for studying biomarkers, tumor growth, drug resistance and mechanism of action in a controlled environment.
Since tumoroids preserve tumor heterogeneity, they provide a more relevant model for testing drug efficacy and toxicity than traditional 2D cultures, enhancing the search for promising drug candidates or the most suitable treatment.
Presently, Oncosyne is focusing its clinical investigation on predictive diagnostics for better treatment of colorectal and pancreatic cancers but aims to continuously expand the iCAN platform to include more tumor types.
Oncosyne’s tumoroid platform iCAN is a
game changer in the fight against cancer
This ground-breaking innovation is based on three novel and differentiating
aspects that distinguish it from other commercially available platforms.
Technical lead
iCAN integrates several key technical and methodological enhancements at all levels, offering substantial improvements in versatility, speed, scalability and increased data precision, accuracy, reliability and reproducibility.
Machine learning
An automated multiplex staining protocol combined with a proprietary digital image analysis algorithm employing supervised machine learning of morphology and functional markers to identify the drug’s mechanism of action substantially improves the assay’s predictive accuracy.
Clinical transition
A multimodal drug prioritization algorithm that integrates existing biological, technical, and clinical knowledge facilitates accurate and reliable clinical translation of in vitro data.
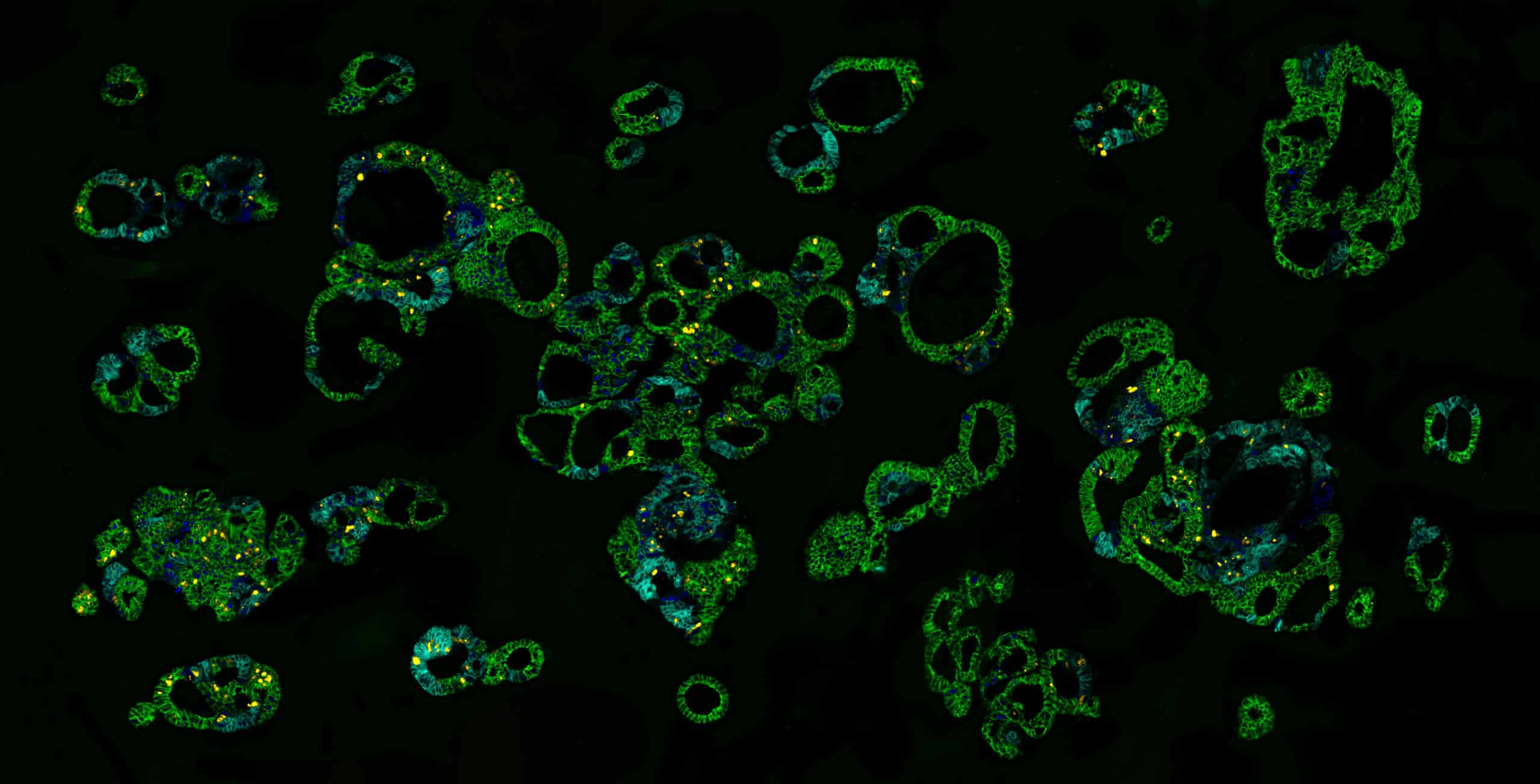
Tumoroids offer unique potential to improve cancer treatment
Various studies exploring in vitro drug screening and predictive diagnostics showcase the immediate clinical relevance of tumoroid-based platforms1-6.
iCAN – data you can trust
A large body of evidence shows that most scientific findings cannot be reliably reproduced or translated—an issue especially pronounced in cancer research7. This reproducibility crisis stems largely from insufficient scientific rigor, publication pressure, and the widespread use of preclinical models that poorly reflect human disease. Conventional cell lines often lack physiological relevance and fail to capture patient heterogeneity, and pharmacological data are often inconsistent when studies are not performed under rigorous conditions8-10. Similarly, reproducibility in animal studies remains very low, as highlighted by the ARRIVE guidelines work11.
Because industrial drug development often builds upon such fragile foundations, the consequences are profound12-13—delayed timelines, failed clinical trials, and in some cases, company failure. The economic losses are immense14, and the human cost for patients awaiting effective treatments is immeasurable.
At Oncosyne, we take this challenge seriously. We believe that meaningful progress in oncology depends on one non-negotiable principle: data quality. Our mission is to help develop new drugs and diagnostic tools that truly work—built on scientific precision, robust methodology, and reproducibility. We apply stringent quality controls at every stage of our work.
Our team brings together over 100,000 hours of combined wet- and dry-lab experience in preclinical modeling and functional analysis. Through this, we have built iCAN, a vertically integrated technology platform representing the culmination of numerous innovations across the laboratory and technology stack. The result is a system that consistently delivers data of the highest scientific and clinical quality—forming a solid foundation for predictive drug development and diagnostics.
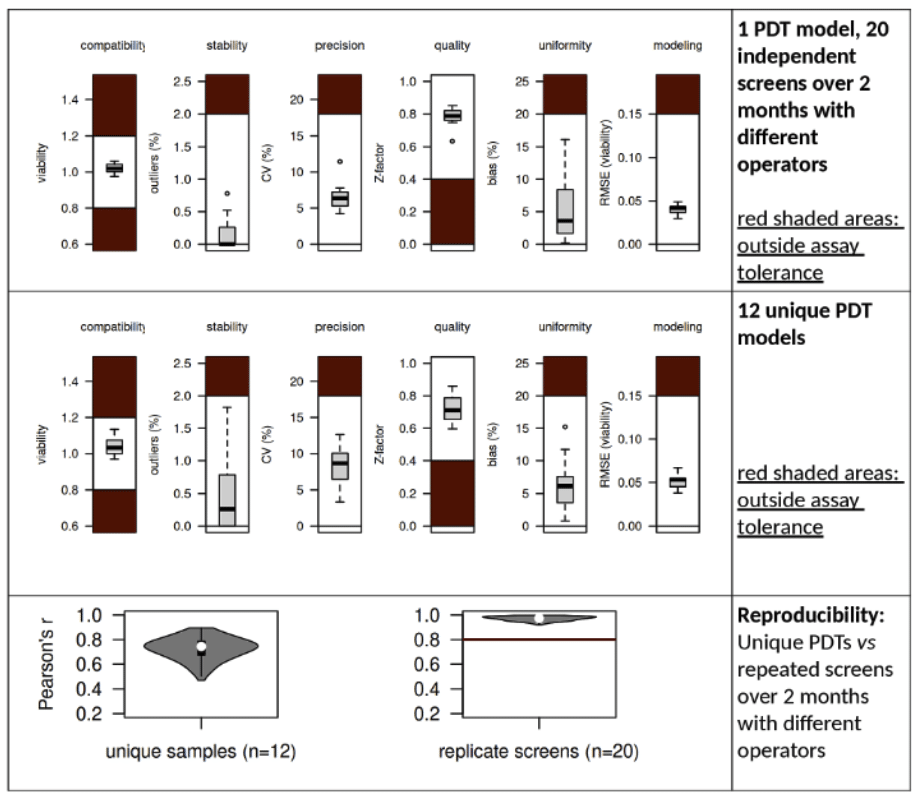
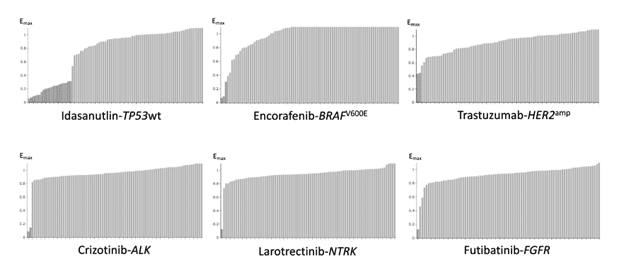
Key publications:
- Bruun, J. et al. Patient-Derived Organoids from Multiple Colorectal Cancer Liver Metastases Reveal Moderate Intra-patient Pharmacotranscriptomic Heterogeneity. Clinical Cancer Research 26 (15): 4107–4119 (2020).
- Ooft, S. N. et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Science Translational Medicine 11, eaay2574 (2019).
- Driehuis, E. et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. PNAS 116, 26580–26590 (2019).
- Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018).
- Tiriac, H. et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discovery 8, 1112–1129 (2018).
- Smabers, L. P. et al. Patient-derived organoids predict treatment response in metastatic colorectal cancer. Clinical Cancer Research Sep 22. doi: 10.1158/1078-0432.CCR-25-1564 (2025).
- Mullard, A. Preclinical cancer research suffers another reproducibility blow. Nature Reviews Drug Discovery Feb; 21(2):89 (2022).
- Safikhani, Z. et al. Revisiting inconsistency in large pharmacogenomic studies. F1000Res Sep 16:5:2333 (2016).
- Haverty, P. M. Reproducible pharmacogenomic profiling of cancer cell line panels. Nature May 19;533(7603):333-7 (2016).
- Niepel, M. A Multi-center Study on the Reproducibility of Drug-Response Assays in Mammalian Cell Lines. Cell Systems Jul 24;9(1):35-48.e5. (2019).
- Song, J. Twelve years after the ARRIVE guidelines: Animal research has not yet arrived at high standards. Laboratory Animals Apr;58(2):109-115 (2024).
- Begley G. C. and Ellis L. M. Drug development: Raise standards for preclinical cancer research. Nature Mar 28;483(7391):531-3 (2012).
- Prinz, F. Believe it or not: how much can we rely on published data on potential drug targets? Nature Reviews Drug Discovery Aug 31;10(9):712 (2011).
- Friedman, L. P. The Economics of Reproducibility in Preclinical Research. Plos Biology Jun 9;13(6):e1002165 (2015).