Clinical Studies
Oncosyne is committed to developing better cancer treatments through close collaboration with leading clinicians and researchers. Below are some of our key ongoing clinical projects.

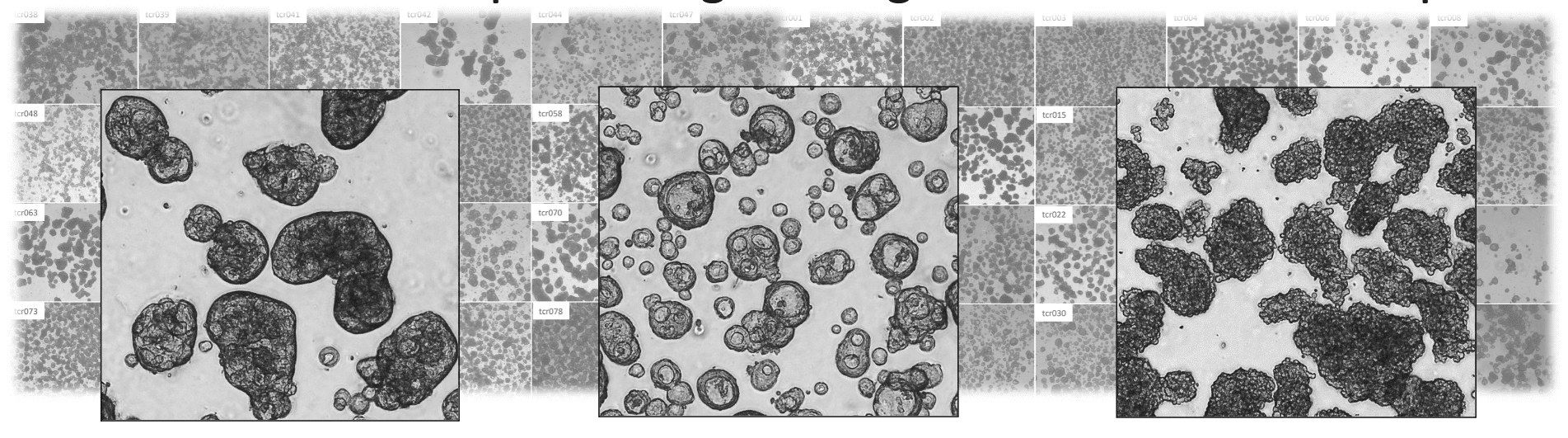
Tailoring Treatment in
Colorectal Cancer (TargetCRC)
ClinicalTrials.gov ID NCT05401318
This study will develop a robust ex vivo platform for personalized drug testing using fresh colorectal cancer samples. By functionally profiling patient tumors against chemotherapies and CAR T-cell therapies, the project aims to identify predictive biomarkers of immunotherapy response.
This approach has the potential to uncover effective combination strategies and extend the benefits of immunotherapy to a large subgroup of colorectal cancer patients who are currently unresponsive to treatment.
TargetCRC is a collaboration between Akershus University Hospital, Oslo University Hospital and Oncosyne.
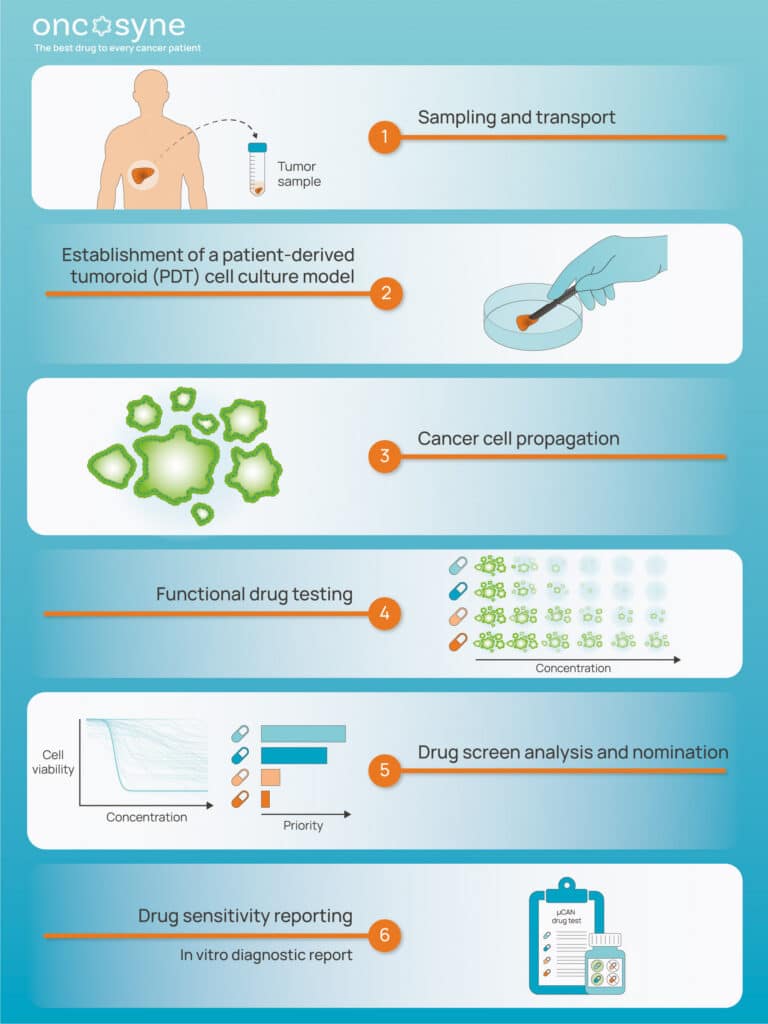
Evaluation of a Diagnostic Test to Identify the Best Drugs for Treatment of Metastatic Colorectal Cancer (DSEE-CRC)
ClinicalTrials.gov ID NCT07171554
DSEE-CRC is a top-tier Norwegian and Swedish public-private partnership for the development of µCAN, a unique patient-centric, therapy-guiding in vitro diagnostic test to improve cancer treatment outcomes for metastatic colorectal cancer patients.
µCAN takes a cancer biopsy sample as input and combines proprietary patient-derived tumoroid culturing conditions with state of-the-art machine learning, and computer-vision guided fluorescence high- content drug screening and analysis, to identify the best therapeutical approach for clinical practice.
DSEE-CRC will have a positive societal and financial impact and directly contributes to the Good Health and Well-being Sustainable Development Goals by delivering patient-tailored treatments, concurrently increasing cancer survivability rates, improving patients’ quality of care, and reducing cancer treatment costs for healthcare providers.
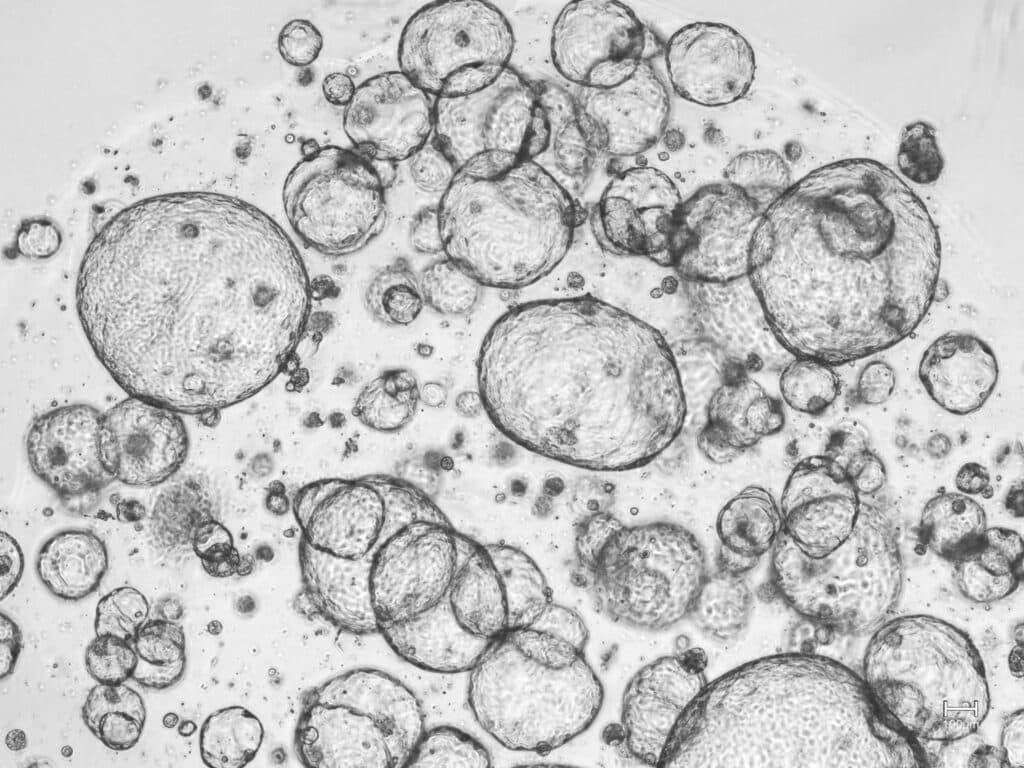
Clinical feasibility of in vitro diagnostic drug testing for pancreatic cancer (INVICTA)
– Integrating the tumor microenvironment for functional precision oncology
INVICTA aims to expand the iCAN platform to enable clinical-grade functional analysis of patient-derived tumoroids from pancreatic cancer. The project will investigate how key components of the tumor microenvironment influence pharmacological responses by integrating cancer-associated fibroblasts, endothelial cells, and biophysical parameters such as extracellular matrix stiffness, hypoxia, shear forces, and interstitial pressure.
To achieve this, a custom organ-on-a-chip microfluidic co-culture device will be developed to more accurately model the tumor microenvironment ex vivo. The diagnostic performance of the platform will be validated through a co-clinical trial and a randomized clinical study, paving the way for its clinical implementation in pancreatic cancer precision medicine.
The study is a collaboration between Sahlgrenska University Hospital, the Hybrid Technology Hub at Oslo University and Oncosyne.
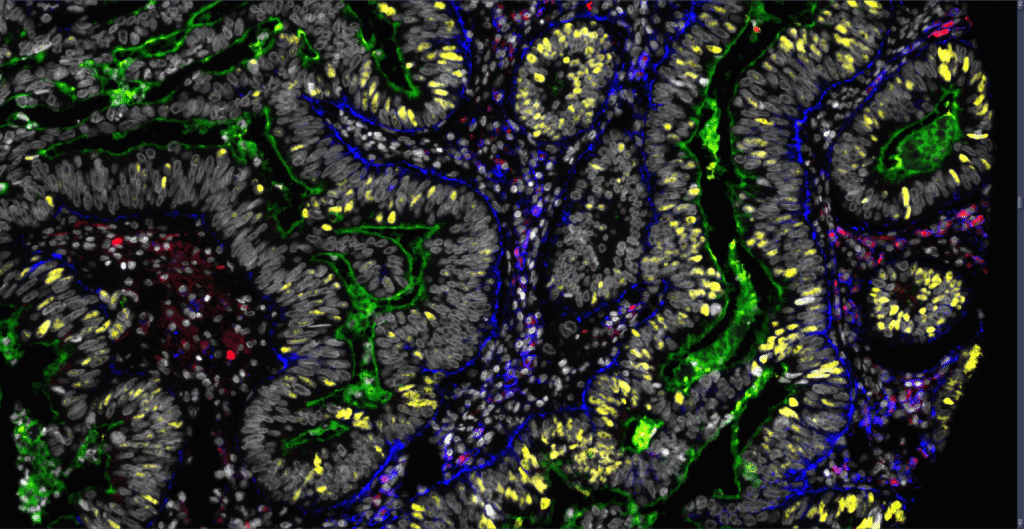
Functional PRecision Oncology: Generating Optimal Circumferential RESection Margins in Rectal Cancer Surgery (PROGRESS)
PROGRESS aims to determine how tumor downstaging influences circumferential resection margins (CRM) and patient outcomes in rectal cancer. Using patient-derived tumoroids (PDTs) that mirror individual tumors to predict the effect of chemoradiation, the project investigates links between clinical downstaging and in vitro therapy sensitivity.
By integrating a rectal cancer database with a national PDT biobank and clinical network, PROGRESS seeks to identify patients who benefit most from neoadjuvant therapy and guide more personalized treatment strategies.
The study is a collaboration between Akershus University Hospital, Haukeland University Hospital and Nordlandssykehuset.
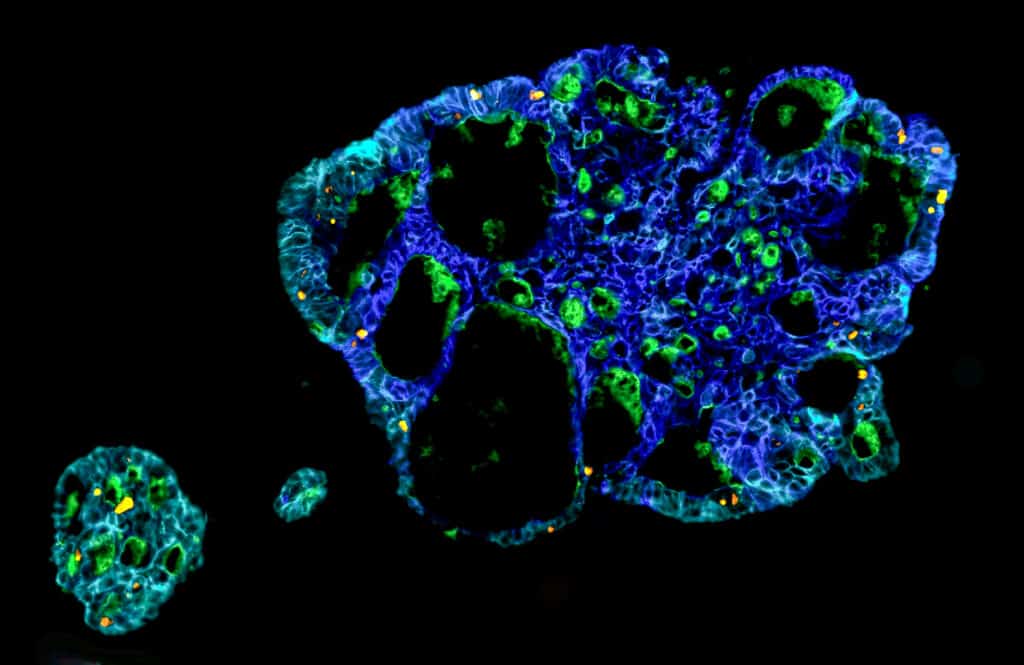
Intra-tumor pharmacological heterogeneity in primary colorectal cancer
This project investigates the extent and nature of pharmacological heterogeneity within primary colorectal cancer through functional profiling of patient-derived tumoroids from distinct tumor regions.
By integrating functional response data with clinical characteristics, treatment outcomes, and survival, the study aims to elucidate how intra-tumor variability influences therapy efficacy and the extent of sampling necessary to capture clinically relevant pharmacological heterogeneity.
The results will provide critical insights into the clinical feasibility of implementing functional precision oncology in the primary setting.