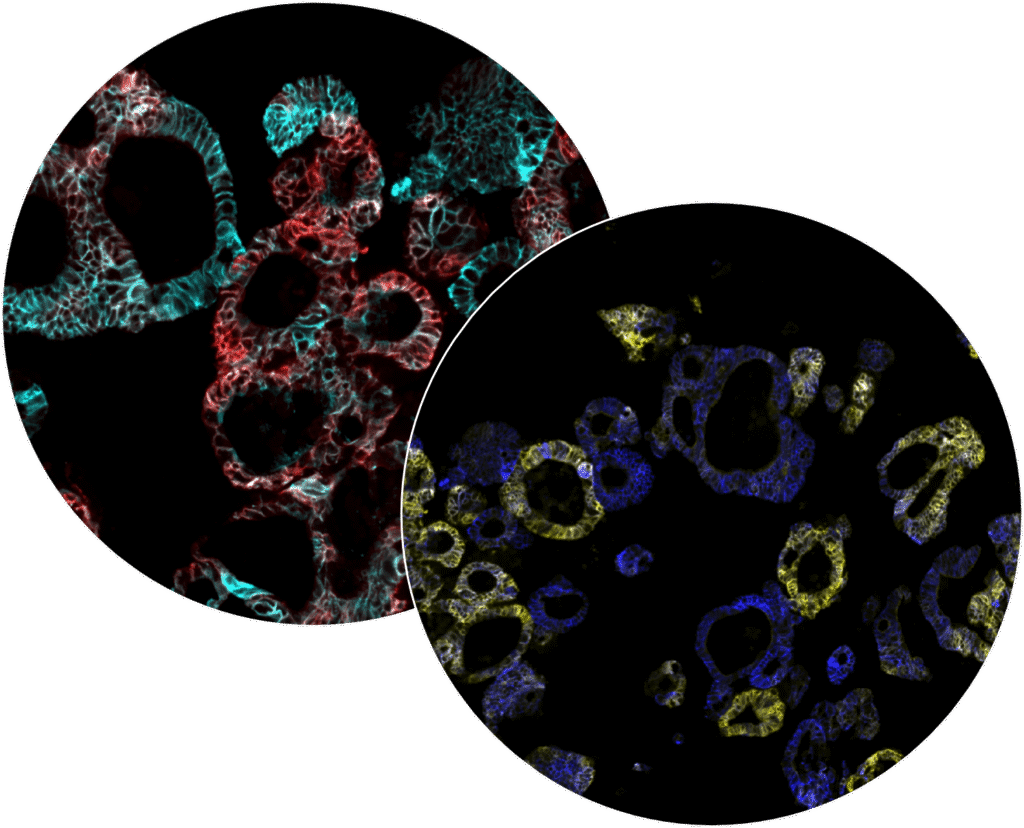
iCAN drug development
Only 6% of oncology drugs entering phase-1 trials ultimately receive FDA approval. The iCAN platform combines high-content clinical-grade drug screening with patient relevant and representative tumoroid models, maximizing clinical translation.
Our drug development services
Creates actionable data of excellent quality for all phases of pharmaceutical development, from discovery to patient stratification in clinical studies. iCAN will accelerate and de-risk your drug development.
“Oncosyne’s iCAN platform has been instrumental in advancing our clinical development of arfolitixorin. The organoid-based experiments not only confirmed the findings of the phase 3 AGENT trial but also provided novel insights into arfolitixorin’s mechanism of action, optimal drug combinations, and potential predictive biomarkers.
Importantly, I believe these data played a key role in supporting discussions with regulatory authorities and ultimately helped us secure approval for our new phase 1b/2 dose-optimization study (NCT06922383).”

Roger Tell
Chief Medical Officer, Isofol Medical
The underlying technology
iCAN tumoroid platform
The iCAN tumoroid platform differs from other commercially available platforms and provides a unique model for testing and analyzing drug activity and mechanism of action in searching for promising drug candidates or the most suitable treatments.
Patient-derived tumoroids
Patient-derived tumoroids are living 3D models of a patient’s tumor in the lab. These tumor twins retain key characteristics of the original cancer, allowing for studying biomarkers, tumor growth, drug resistance and mechanism of action in a controlled environment.
Recent news
-
Oncosyne hosts a project seminar with Sahlgrenska to advance pancreatic cancer care
As part of the INVICTA project, Oncosyne recently hosted a project seminar with clinical collaborators from Sahlgrenska University Hospital. The meeting was held at Oslo Cancer Cluster and focused on how our tumoroid-based technology can support improved treatment outcomes for patients with pancreatic cancer. The project is progressing well and we are well on track…

iCAN healthcare
Today’s standard methods predict cancer treatment outcomes poorly, leading to 70% of patients receiving suboptimal care. iCAN diagnostics using patient-derived tumoroids personalizes optimal treatment for every patient.
Our diagnostic tests
Oncosyne’s first test for colorectal cancers is being validated in an ongoing clinical performance study. Oncosyne’s test for pancreatic cancers is under development.
